With the recent completion of the new commercial water softeners, Wedgefield customers should be seeing some welcome changes in their water quality. The
introduction of this softening system will provide a water with significantly less calcium hardness. There are many benefits to softer water:

- You use less soap – up to 75% less, saving money on cleaning products and reducing their impact on the environment.
- Your plumbing lasts longer – calcium scale can clog pipes and valves and reduce your water pressure.
- Your hot water heater and appliances will last longer – scale deposits not only shorten the life of your water heater and any appliance that heats water;
the build-up of deposits can increase the amount of energy used to heat your water.
- Your skin and hair feel softer – many people rinse with lemon juice to get mineral build-up out of their hair, with soft water you won’t have that
build-up!
- Your dishes will have less spots and your sinks, showers and toilets will have less soap scum build-up.
All these benefits will save money and time. Since the majority of Pluris customers do not have residential water softeners, they will see the benefit from
the new water softeners installed by Pluris. And customers with residential water softeners will no longer need their own residential water softeners,
which will also save them considerable money, as they will no longer have to pay for salt or to maintain the units.
So what makes a water hard or soft? After softening, the water actually will feel “softer” on your skin, some say that is the origin of the term soft
water. Others note it is called hard water because it comes from the ground, which is hard, whereas soft water comes from rain. Either way, the amount of
minerals, mainly calcium (Ca) and magnesium (Mg), that are dissolved in water determine the relative hardness and whether it is considered hard or soft.
Excessive hardness in water leaves scale deposits on you sink, your skin, your water heater, etc. This scale is mostly in the form of calcium carbonate
(CaCO3). Water with hardness of 0-60 milligrams per liter (mg/l) (measured as CaCO3) is considered to be relatively soft water.
Moderately hard water is considered to be between 60-120 mg/l, while very hard water has hardness of 150 mg/l and up.
Softeners, like the system recently installed by Pluris, reduce the amount of hardness in the water. Prior to softening, Pluris water has a hardness of 220
mg/l, it is very hard. After softening it is <50 mg/l, so the treated water is now considered soft.
However, it is important to recognize that some hardness is a good thing. If all of the minerals are removed, such as occurs when water is treated by
reverse osmosis or over-softened, the water may become corrosive. Corrosive water will attack the metal components in residential plumbing and appliances.
A corrosive water condition can also result in problems from lead and copper that can leach out of plumbing fixtures.
To prevent water from being either too corrosive or creating too much scale, utilities work to keep the water in balance. This is done by balancing the pH
and alkalinity to produce a stable, non-corrosive water. The pH is a measure of the acidity of a solution. The pH scale ranges from 0 to 14. A pH of 7 is
considered neutral. Water below 7 is considered acidic, water above it is called basic or alkaline. Some amount of alkalinity, in the form of hydroxides,
carbonates and bicarbonates (think Alka-Seltzer) should remain in the water to absorb acids and prevent corrosive conditions. Alkalinity is measured as
calcium carbonate (CaCO3) in laboratory testing, and is an indicator of calcium hardness.
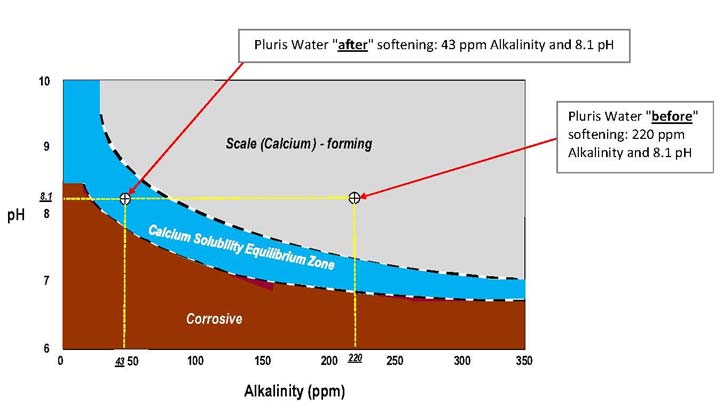
One method of determining whether the pH and alkalinity are properly adjusted to produce stable, non-corrosive water is with the Baylis Curve, as shown
below. Water that falls in the upper zone will cause excessive scale, water in the lower zone will be corrosive. Our goal is to produce water in the
equilibrium zone, where there is enough alkalinity to keep the water stabilized but not too much to cause scaling.
As can be seen on this figure, before softening, water withdrawn from central Florida wells is quite hard. The original Wedgefield water treatment plant
constructed in the 1960s did not provide any water softening treatment. Water softening at the plant did not occur until 2009, when the prior utility
owners installed water softeners at the facility. However, these softeners did not consistently reduce hardness to an effective level. As a result, for
over 40 some years the majority of customers, and our distribution system piping, have experienced the effects of the scale build up, as well as soap scum,
on appliances, cookware and glassware.
The new commercial water softeners installed by Pluris are designed to substantially reduce the hardness and will do so on a consistent basis 24/7/365.
Water testing of the alkalinity has averaged 43 ppm since start up at the end of October, 2015 and customers have commented on the improved water quality.
As noted, water that is too soft may cause issues with corrosion. Pluris customers with residential water softeners may want to consider no longer
keeping their softeners. For a period of time, customers desiring to know the present actual hardness in their home are encouraged to contact Garth
Armstrong, Project Manager for Pluris at [email protected]. Mr. Armstrong will schedule a meeting
at the customer’s residence and bring a Chemist with Flowers Chemical Laboratories, Inc., a state of Florida NELAC certified laboratory to provide 3 rd party testing for hardness.
The above article provided by Kiera S. Fitzgerald, PE, Principal Engineer with D.P. Fitzgerald, Inc., an engineering firm in Alachua, Florida,
specializing in water and wastewater treatment design. Ms. Fitzgerald has over 30 years in the water and wastewater utility industry. For information
regarding the article, readers are encouraged to contact Ms. Fitzgerald at
[email protected]
.

