The America Water Works Association (“AWWA”), in its November Journal features Pluris’ chlorine dioxide treatment process at its advanced water treatment plant near Orlando, Florida. The article details Pluris’ decision three years ago to use chlorine dioxide as an alternative disinfectant to chlorine in producing safe drinking water while reducing disinfectant by-products related to chlorine. The article summarizes the over two years of successful treatment since the conclusion of the Florida Department of Environmental Protection authorized pilot study, with total trihalomethanes (“TTHM”) being non-detected in drinking water. The article is presented below in full.
For more information readers are encouraged to contact Joe Kuhns, Regional Manager for Pluris in Florida. Joe can be reached at [email protected] or at his office at (863) 940-9771.
Consider Chlorine Dioxide to Reduce Disinfection Byproducts
With the challenge of meeting the current Stage 2 and future maximum contaminant levels, water utilities are seeking treatment technologies to ensure ongoing compliance. The detection of elevated levels of total trihalomethanes prompted a Florida water treatment plant to conduct a successful pilot study using chlorine dioxide for disinfection.
BY LANCE LITTRELL, STEVE ROMANO, RHEA DORRIS, GINA PARRA, MAURICE GALLARDA, AND JOE KUHNS
CHLORINE IS one of the most commonly used disinfectants today for potable water, but it’s increasingly subject to criticism because it reacts with organic matter to form potentially carcinogenic disinfection byproducts (DBPs). Alternatively, chlorine dioxide (ClO2) is a strong and selective oxidant offering disinfection without the halogenated DBPs.
ClO2 has been used for water treatment since the 1970s after the discovery of total trihalomethanes (TTHM). It’s being used at approximately 1,200 facilities in Europe and the United States, and its application can be tailored to a specific facility’s needs. Also, the compound can be used as the primary disinfectant or as a preliminary oxidant followed by chlorine or chloramines. ClO2 reactions in water don’t form TTHM and haloacetic acids (HAA5) because, as described in a July 2017 Journal AWWA article by G.W. Holden (https://doi.org/10.5942/jawwa.2017.109.0089), as ClO2 oxidizes organic material, it’s reduced to chlorite, but it doesn’t chlorinate the resulting organics. ClO2 has been shown to have five times stronger oxidation potential and disinfection efficacy than chlorine. Also, the US Environmental Protection Agency identifies ClO2 as an acceptable method of inactivating viruses and bacteria to achieve 4-log virus inactivation.
CHLORINE DIOXIDE PILOT STUDY
These are some of the reasons Pluris, which owns the Wedgefield (Fla.) Water Treatment Plant, conducted a comprehensive chlorine dioxide pilot study. With the onset of the Stage 2 Disinfectants and Disinfection Byproducts Rule (DBPR), the Wedgefield facility attempted to maintain compliance with the DBPR. In 2016, the TTHM samples exceeded the 80 parts per billion (ppb) regulatory limit as a single sample and approached the annual rolling four-quarter average limit. The utility streamlined the chlorine dosage to reduce the residual concentration but was unable to achieve TTHM below 80 ppb. As a result, the utility sought alternative treatment methods to achieve regulatory compliance. It opted not to consider chloramine disinfection because of chloramine’s own distribution system challenges.
Preliminary Analysis, Testing, and Goals.
Through field testing and laboratory evaluation of ClO2 products, the plant decided to implement a full-scale, systemwide pilot test. Field testing efforts included demand analysis testing of the treated water for demand curve identification. Laboratory testing simulated ClO2’s use as a preoxidant to chlorine

disinfection. This testing was completed by the University of Central Florida’s Environmental Systems Engineering Institute (UCF ESEI) and revealed preoxidation wasn’t suitable. Further testing was conducted to simulate ClO2 as the primary disinfectant. The results proved positive in support of a full transition to ClO2 to reduce TTHMs within the system.
The next step in the process was to demonstrate the laboratory effects on the full-scale storage and distribution system. The goals of the full-scale pilot study included a transition from chlorine disinfection to ClO2; vigorous field and laboratory testing to ensure public safety; and regulatory compliance, including 0.05 ppm residual, 0.8 ppm maximum residual disinfectant level (MRDL), and 1 ppm maximum contaminant level (MCL) for chlorite. A pilot testing approval package was approved by the Florida Department of Environmental Protection (FDEP), and the pilot test was initiated. ClO2 was injected into the ground storage tank in parallel with the chlorine disinfectant dose. ClO2 residual was monitored in the distribution system. Once the ClO2 residual was attained, chlorine dosage was trimmed slowly to perform the gradual disinfection transition. As chlorine was reduced, continuous monitoring of the ClO2 residuals ensured compliance.
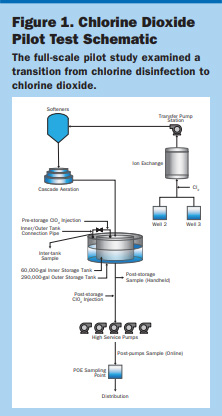
Pilot Overview. The Wedgefield Water Treatment Plant includes two raw water wells feeding directly into the ion-exchange system, which removes approximately 60 percent of the total organic carbon (TOC) and approximately 95 percent of hydrogen sulfide (H2S). The treated water is softened prior to tray aeration and storage. High-service pumps distribute finished water for potable use.
The pilot study (Figure 1) was designed to inject a premixed 0.3 percent ClO2 solution into the ground storage tank downstream of the tray aerators to use the H2S removal and downstream of the softeners to prevent any oxidation of the softening media. After storage, ClO2 was monitored via a handheld analyzer as well as an online analyzer for continuous readings as the water entered the distribution system. Additional monitoring in the distribution system was completed using the handheld analyzer.
Process Optimization. Utility staff closely monitored the residuals as the transition to ClO2 extended through the distribution system. The ClO2 dosage was adjusted to maintain the desired 0.05 mg/L distribution system residual. ClO2 residual was monitored throughout the distribution system to ensure it had reached the distribution system extents prior to discontinuing the chlorine injection. The initial dosage rate fluctuated at the beginning of the pilot as the distribution system accepted the transition.
The operations staff monitored continuously and recorded the ClO2 levels at least twice a day. The handheld probes confirmed the online ClO2 residuals, which were used to confirm and regulate the feed rate of the ClO2.
As with any pilot study, the planned operation rarely follows the scripted procedure. The utility and its hands-on operations team were instrumental in its success. Once the chlorine dioxide was introduced, the chlorine residual quickly began to rise within the distribution system. As the ClO2 dose increased, the operation staff adjusted chlorine to account for the decreased chlorine demand. The pilot study data showed that the stronger oxidant reacted with the system demand, requiring a slight chlorine dose to achieve the 0.2 ppm residual during the transition’s initial phases. As the ClO2 achieved the minimum residual required
for the distribution system, the chlorine dosage was subsequently eliminated. Chlorine presence in the water can reduce chlorite formation, so the utility operations staff used a small dosage pump to gradually reduce chlorine while carefully monitoring chlorite during the disinfection transition.
STUDY RESULTS
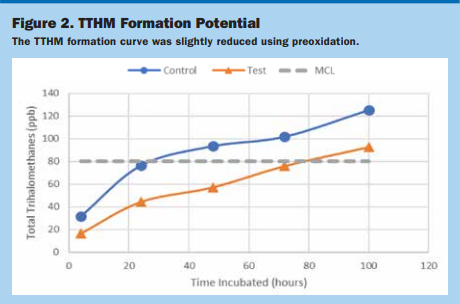
Preoxidant Evaluation. The bench-scale and laboratory testing revealed the ClO2 inhibits or reduces the formation of chloroform TTHM species when dosed prior to chlorine, whereas the bromoform TTHM species were unaffected. With the plant’s TTHM speciation driven mainly by brominated species, preoxidation was ruled out for this facility following the UCF ESEI analysis. Figure 2 shows that the TTHM formation curve was slightly reduced using preoxidation because of chloroform reduction.
TTHM Reduction. The pilot study results confirmed the combination of ClO2 and chlorine as dual disinfectants effectively reduced TTHM concentrations. Additionally, ClO2 as a primary disinfectant proved to be successful in eliminating TTHMs after the transition from chlorine was completed.
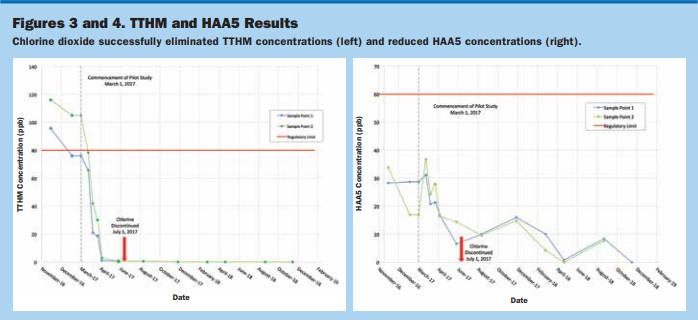
Figure 3 displays the TTHM sampling events during the pilot study and the two compliance samples before initiating the pilot study. TTHMs declined from more than 110 to 20 ppb (about two months after commencement) and were nondetectable by June 2017, near the end of the 180-day pilot. TTHMs have remained undetectable since June 2017 (more than two years), and the FDEP has approved DBP testing reduction to annual events.
HAA5 Reduction. HAA5 concentrations, although at a lesser rate than that of TTHMs, also decreased during the study. Figure 4 shows the two previous HAA5 compliance samples prior to the commencement of the study along with
Careful consideration should be given to
ClO2 implementation within a water
production and distribution system.
compliance testing through the latest compliance testing.
REGULATORY COMPLIANCE
It was imperative that all regulations were met, so special attention was given to the ClO2 MRDL and the chlorite MCL.After initial fluctuations during early operation, a new control system was implemented to provide more accurate monitoring. The full-scale system has stabilized and maintains consistent readings from the online analyzer.
Meeting Chlorite Residual Standards. Laboratory results and field analysis samples taken with the handheld analyzer confirmed chlorite levels were maintained below the MCL of 1 ppm throughout the pilot study. The chlorite concentration ultimately leveled off at 0.6–0.8 ppm.
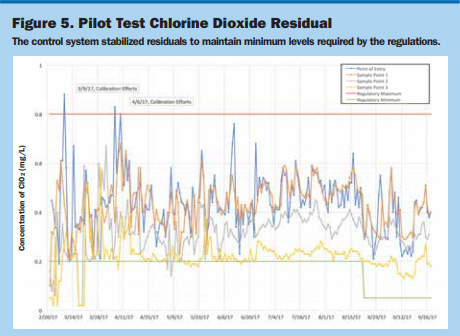
Meeting Chlorine Dioxide Residual Standards. Daily data from the study’s ClO2 residual results are shown in Figure 5. The residual dropped below the 0.2 ppm residual at the beginning of the pilot. There were only two exceedances at the point-of-entry analyzer, which were the result of erratic readings during calibration events. The control system stabilized residuals to maintain minimum levels required by the regulations.
TRANSITIONING TO CHLORINE DIOXIDE
The study’s goal was to reduce DBP formation within the Wedgefield community and maintain the plant’s consistent regulatory compliance. The study period data revealed 99 percent and 50 percent reduction in TTHM and HAA5, respectively. Two additional years of data revealed 100 percent and 89 percent reductions in TTHMs and HAA5s, respectively. As a disinfectant, the plant hasn’t experienced any adverse issues in the distribution system because of the transition from chlorine to chlorine dioxide.
ClO2 proved highly effective at minimizing DBP formation without the high capital costs of treatment upgrades. ClO2 is relatively uncommon, so Pluris believed it was important to fully understand the process before investigating its use. The following recommendations are based on lessons learned from the pilot study.
- ClO2 effectiveness of Stage 2 DBPR compliance is proven, but due to variability in source water quality, investigative field and laboratory testing is recommended to verify treatment compatibility. Additionally, a full-scale and/or pilot-scale study is recommended to identify the best application (preoxidation or disinfection), optimize dosage, and confirm chlorite MCL compliance.
- Coordination before and during pilot testing with state and local regulators is important to ensure ongoing compliance with all water quality regulations.
- Understanding and reviewing available options for ClO2 generation are imperative. These include operator training and availability, ClO2 goal usage, redundancy, process compatibility, and safety.
- Proactive and direct public communication is important in emphasizing the benefits of ClO2 and in comparing the safety of ClO2 with other disinfectants.
EXPANDING CHLORINE DIOXIDE’S USE
Careful consideration should be given to ClO2 implementation within a water production and distribution system. Although the chemical effectively maintains disinfectant residuals and improves aesthetics in distribution system water quality, the appropriate process addition may be as a preoxidant rather than as the primary disinfectant.
ClO2 has shown promise as a strong disinfectant chemical for other utilities aspiring to reduce DBPs without incurring significant capital cost associated with alternative treatment or the routine maintenance challenges with chloramines. As a viable alternative disinfectant, it should be considered when these DBP or distribution system challenges are present.
Acknowledgments: Special thanks to those who made this project possible: Pluris Wedgefield’s corporate and operation staff members, who supported the application and provided extensive hands-on testing analysis for the data collection and continuous analysis of the field data; Twin Oxide-USA; and the Florida Department of Environmental Protection staff.

